 A DEXA scan supposedly evaluates bone health. It doesn’t. It seems more like smoke and mirrors. Here’s what you should know.
A DEXA scan supposedly evaluates bone health. It doesn’t. It seems more like smoke and mirrors. Here’s what you should know.
BEFORE READING ABOUT MY ‘OLD’ BONES … ARE YOU, LIKE ME, A SENIOR WHO’S INTERESTED IN STAYING HEALTHY FOR YEARS TO COME? IF SO, YOU MIGHT LIKE TO SEE WHAT A SCIENTIST (ME) HAS TO SAY ABOUT HOW TO ACHIEVE IT AT NO EXTRA COST TO YOU, WITHOUT EVEN HAVING TO LEAVE HOME, STARTING HERE: HEALTHY AGING NATURALLY.
The DEXA Scan Myth
HEADS UP BEFORE WE GET STARTED: This post has some pretty geeky stuff about what a DEXA scan does and what it means. So I’ve decided to state the main issue right up front here. Then, if you’re really interested in what you can expect from such a scan, and what to do if you get ‘scary’ results from one, please continue to read about my experience and what I learned from it.
The core of the myth: a DEXA scan (Dual-energy X-ray absorptiometry) for bone density tells you something about bone strength.
Regardless of what your doctor or anyone else says, bone density and bone strength are not the same thing.
Nevertheless, results of a DEXA scan can be used for justifying prescriptions for bisphosphonate drugs (e.g., Foxamax, Boniva, Actonel), which do, indeed, increase bone density.
They do so by preventing old, tired bone cells from being recycled.
Keeping geriatric bone cells around longer does increase bone density.
It’s just that keeping old bone cells around too long can weaken bone structure.
As a result it also increases the risk of bone fractures.
In fact, one of the earliest side effects is weakened jawbones, leading to loose teeth. Thus, the jawbone acts like an early warning system for bone weakening in the rest of your body.
Does that sound like increased bone strength? Nope!
Instead it’s a bit of misdirection for the purpose of prescribing bisphosphonates.
If you’re confused, then simply apply one of my favorite mantras: Follow the money.
Bisphosphonate drugs are a huge moneymaker for drug companies.
Nevertheless…
Researchers long ago established that bone density and bone strength are NOT the same thing.
The following two quotes make that very clear (full references at the end of this post):
From Friedman (2006):
Bone mineral density (BMD) is a useful tool for diagnosis; however, this parameter provides information regarding only the quantity of mineral in bone, which is only one component of bone strength.
From Ott (2016):
This important study has been nearly forgotten, but it remains the most clear-cut evidence that bone density is not the same as bone strength.
SIDENOTE: The Ott study focused on the consequences of fluoride for increasing bone density, which is accompanied higher incidences of bone fractures. Fluoride is evil in a lot of ways. This is just one of them.
Ultimately, this means you have to keep your BS detector set on ‘high’ when medical folks insist on a DEXA scan to evaluate your bone health.
Regardless, we’re still stuck with DEXA scans.
Why?
Well, they’re cheap, fast, non-invasive – and covered by insurance for us ‘mature’ folks (i.e., Medicare, which pays for one every two years).
Eventually you’re likely to have a DEXA scan. Just about everyone does.
So what, if anything, can you actually get out of it?
Here’s what I found.
Doing the DEXA Scan Two-Step
At the end of my recent annual physical, my PA (almost never see the doctor) suggested I get a DEXA scan.
The test itself was simple and fast – 10 minutes or so.
My results were, shall we say, interesting.
So was the advice I got about them. I’ll get to that later.
It was the final impetus for me to satisfy my curiosity about what a DEXA scan is and what it can tell me about my bones.
That’s when the ol’ DEXA scan two-step got started.
Unfortunately, what I discovered is a land of medical doublespeak designed to scare me into taking a prescription drug.
What exactly is this doublespeak?
Regarding bone health, conventional medicine wants you to believe a DEXA scan can tell you whether your bones are weakening.
“It measures bone loss,” says Dr. Bart Clarke, a Mayo Clinic endocrinologist. “It tells us how strong the bones are even if they haven’t broken yet.”
In other words, Dr. Clarke’s statement is completely incorrect.
It’s a logic two-step that seems to be lost on the majority of medical folks, in spite of plenty of scientific literature showing the two are NOT the same thing.
Understanding DEXA SCAN Results
Okay, let’s dig into what a DEXA scan actually tells you.
First off, the actual measurement is called bone mineral density (BMD). It’s expressed in milligrams per square centimeter.
Ignore for the moment that density is a 3-D measure, whereas the data are expressed in 2-D, i.e., square centimeters. A true density would be in cubic centimeters, or cc.
A BMD is typically taken at three different locations – lumbar spine, left femoral neck, and total hip.
My BMDs were then used for calculating my T-scores and Z-scores.
I’ll get to those scores a little later.
Meanwhile, this is how my results were listed on my DEXA scan report:
LUMBAR SPINE (L1-L4):
BMD: 1.060 g/cm2
T-Score: -1.3
Z-Score: -0.8
LEFT FEMORAL NECK:
BMD: 0.853 g/cm2
T-Score: -1.7
Z-Score: -0.3
LEFT TOTAL HIP:
BMD: 0.926 g/cm2
T-Score: -1.3
Z-Score: -0.3
Although numbers can be fun, my question was how those results stacked up. I.e., were they ‘normal’? The numbers themselves told me nothing.
Therefore, for comparison, I dug up this graph of age-related bone densities, courtesy of the University of Washington.
The red arrow pointing to the red asterisk is my personal datapoint. It’s smack dab in the middle of my age cohort.
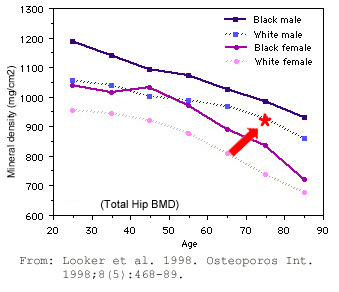
It looks like my BMD is right where it should be for my age group, right?
So far, so good.
WAIT, THERE’S MORE!
What I really wanted to know was my probability for suffering a bone fracture.
Not to worry. That’s exactly what the math geeks have figured out, based on some convoluted calculations. They form the basis for what’s appropriately called a FRAX score.
A FRAX score is the 10-year probability of developing a fracture. It consists of two numbers, one for any fracture and one for a hip fracture.
My two FRAX numbers were:
6.9% for any fracture
2.0% for a hip fracture
Those numbers were followed by this semi-ominous comment: This patient’s bone mineral density is in the range of low bone mass (osteopenia). This implies moderate fracture risk.
Aha! Finally – an official diagnosis! (I feel so much better now.)
Of course, I’m not one to simply accept such a diagnosis without understanding how the numbers are calculated. So I went digging into it to get a better grasp on how it works. And that brought on some new confusion.
Hunting down the explanation led to my discovery that FRAX is a trademarked name – i.e., FRAX®.
It’s owned by the University of Sheffield, England, where it was developed.
This just means that the formula is semi-secret and that clinics using it have to pay for approval to use the name.
Fortunately, the University of Sheffield has an online FRAX Calculation Tool available for free to the public. Calculations can vary by country, so the dropdown menu has dozens of choices. When I chose ‘North America’, a page for ‘US Caucasians’ popped up, oddly enough with no other options.
Interestingly, when I used that tool myself, my FRAX scores increased, to 9.9% for any fracture and 4.4% for a hip fracture. Hmm.
What About Those T and Z Scores?
The T-score is the relevant measure when screening for osteoporosis. It is the bone mineral density at the site when compared to the “young normal reference mean”. It is a comparison of a patient’s bone mineral density to that of a healthy 30-year-old.
The International Society for Clinical Densitometry recommends categorizing the patient according to the lowest T-score from the total L-spine, total hip, and femoral neck, utilizing the following World Health Organization criteria:
Normal range: T-score of -1.0 or above
Osteopenia: T-score of less than -1.0 but greater than -2.5 (mine was -1.3)
Osteoporosis: T-score of -2.5 or below
So, once more, I appear to be on the cusp of >osteopenia, which is defined as low bone density. (Again, NOT low bone strength.)
The Z-score for bone density is the comparison to the “age-matched normal” and is usually used in cases of severe osteoporosis. This is the standard score or number of standard deviations a patient’s bone mineral density differs from the average for their age, sex, and ethnicity. This value is used in premenopausal women, men under the age of 50, and in children and adolescents. It is most useful when the score is less than 2 standard deviations below this normal.
My Z-scores ranged from -0.8 to -0.3. I don’t really know what this means, since I don’t have severe osteoporosis, nor am I in in any of the target groups (premenopausal women, men under the age of 50, children, adolescents).
OK…after all that falderol, what else did my DEXA scan results say?
This curious comment came toward the end of my report:
RELATED INFORMATION:
The T-score is relative to peak bone mass in early adulthood, while the Z-score is relative to individuals of the same age and sex.
Men 50 and older: WHO data is not available for men of this age group. Consequently, data is extrapolated from data for postmenopausal females. T-score data for men over age 50 and older is preferred.
Premenopausal & men under 50: In premenopausal females and men under 50 years of age, Z-scores, not T-scores, are preferred. A Z-score of -2.0 or lower is defined as below the expected range for age and a Z-score above -2.0 is within expected range for age.
Wow, that clears up a lot, right? (NOT!)
Ultimately…
Regardless of all that statistical mumbo-jumbo, I’m left mainly with that graph above, comparing BMD vs. age class.
However, I object to the idea that decreasing BMD is age-related. Comparing populations based on age ignores all the myriad lifestyle choices people make over a lifetime. Dan Buettner’s book, The Blue Zones Secrets for Living Longer: Lessons From the Healthiest Places on Earth shows that super centenarians would blow this curve out of the water.
So it’s not about aging.
Aging bones don’t necessarily have to get thinner. They just happen to do so in the populations sampled for the WHO data, etc.
In other words, it appears to be a modern lifestyle phenomenon, not an age-related phenomenon.
That comment deserves some explanation. Sure, the graph is real. It does show bone loss with advancing age. This kind of correlation inappropriately implies cause-and-effect. In this case, it implies aging is the cause and bone loss is the effect.
Hold that thought for a moment. If your BS detector isn’t sounding off yet, it soon will.
The graph only shows a window in modern times. At best, it’s myopic. To get the real nitty-gritty, we need to take a 30,000-foot view – i.e., over a longer time frame.
A (Pre)-Historical Digression
The time frame I’m talking about is millions of years. Such a long timespan provides a better perspective of what bone densities looked like historically – actually, prehistorically – compared with the present.
The article by Chirchir et al. (2015), cited in the references, does that. In so doing, it compares trabecular bone structure with bone density, which is a better indicator than bone density by itself.
NOTE: As outlined by Shafiee et al. (2020) [see full reference below] … Trabecular bone score (TBS), as a tool for measurement of bone microarchitecture, represents fracture risk independently of bone density.
Comparing TBS among modern humans, prehistoric humans, and related primates reveals a not too surprising thinning of our bone structure over evolutionary time, as shown below.
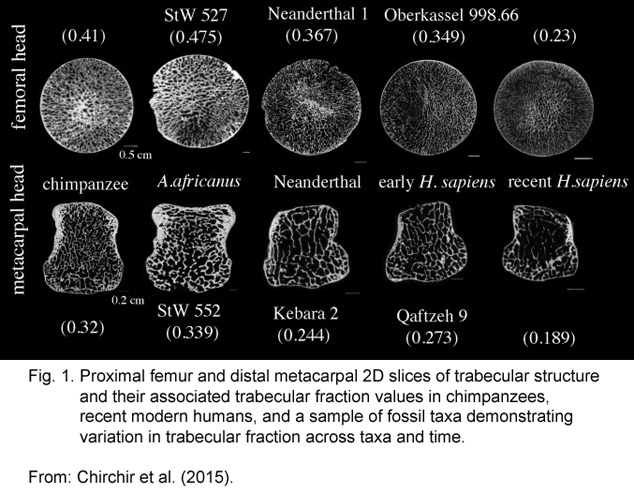
The images show bone trabecular structures in weight-bearing bones in our closest non-human relative (chimpanzee), in pre-human hominids (Australopithecus africanus and Neanderthals), and in early and modern humans (Homo sapiens).
Although the image is a little dark, the trabecular fraction values quantify the differences. Modern humans have the lowest values (femoral at 0.23 and metacarpal at 0.189).
SIDENOTE: If you’re wondering why we don’t use this kind of assay instead of DEXA for evaluating living bone structure, it’s because it requires bone sections – usually only available from deceased ‘volunteers’. In other words, from fossils and autopsies.
What does all this mean? Ultimately…
Thus, the low trabecular density of the recent modern human skeleton evolved late in our evolutionary history, potentially resulting from increased sedentism and reliance on technological and cultural innovations. (Chirchir et al. 2015).
In other words: our ever-thinning bones come to us courtesy of our modern lifestyles.
To be crystal clear, what all that science-y stuff says, in everyday language is:
We are sitting on our butts, watching too much TV or enjoying other “cultural innovations” – i.e., the creature comforts of modern living.
To repeat: Increasing bone loss over time is a lifestyle issue, not an aging issue.
This explains why ALL of the advice I found about maintaining good bone density included the admonition to get moving.
We’re probably not going to revert to our old-time hunter-gatherer behaviors any time soon. However, coming as close to that high-movement lifestyle as you can is a gazillion times better than anything modern medicine has to offer. Stronger bones would be just one of the benefits.
Of course, countless other lifestyle choices play their roles in our bone health. Sedentary behavior is an obvious issue. Diet, too. Nutritional status certainly has a role.
A CAUTION
Activity has its limits.
Medical schools teach future doctors about something called Wolff’s Law. It holds that bone in a healthy animal will adapt to the loads under which it is placed.
This is the premise behind advice for building bone strength through weight-bearing exercise.
It’s why conventional medicine predicts stronger bones in the obese. After all, their bones bear more weight.
The caution is this: Wolff’s Law totally fails in people who are obese due to leptin-resistance. The details on why that’s the case are pretty geeky, so I’ll skip them here.
The bottom line is that obesity is associated with accelerated bone loss. It’s just the opposite of what doctors are taught.
Recommendations for Good Bone Health
Now, after all that statistical hocus-pocus from my DEXA scan, and after taking a peek at what’s possibly behind our modern bone loss, what can you do?
Standard advice, which I received from my PA, consisted of this:
- Supplement with vitamin D (1,000-2,000 a day)
- Supplement with calcium (1,000 mg a day)
- Get more exercise
Regarding vitamin D, my natural level (i.e., primarily from sunshine) was already at nearly 50 ng/mL. That’s already pretty good. I did start supplementing with vitamin D, at 5,000 IU a day, which brought me up to 65 ng/mL. Generally speaking, 30 ng/mL should be the lower limit. (Some populations around the world have been measured at an average of less than 10 ng/mL.)
As for the advice to supplement with calcium … well, that’s pretty standard, too. Unfortunately, it’s not that straightforward. These two earlier posts explain what you should know about it: Why Taking More Calcium Is Not The Best Osteoporosis Treatment and Are Dangers of Calcium Supplements Real?
So, no – I won’t be taking any calcium supplements.
What I did instead is boost my intake of vitamin K2. It’s the key missing vitamin in nearly all recommendations about bone health, including the ones I received.
I also took my own advice, as described in the osteoporosis post linked above, by taking 1,500 mg of strontium daily. I explain more about that mineral and its role for bone health in that article.
Finally, advice to ‘get more exercise’ is also standard fare. However, at this time in my life I’m already getting plenty. In a typical week I do one weight-training resistance workout, two interval workouts, two yoga classes, and one or two rounds of golf.
And I pay attention to avoiding one of the latest bugaboos modern lifestyle choices – sitting disease. Yes, it’s a real thing. I provide some pointers about it in this earlier post: Newest Weight Loss Methods Tackle Sitting Disease.
In other words, the advice I got based on my DEXA scan – and my supposedly incipient ostepenia – was pretty useless for me personally.
Nevertheless, following my activity and supplementation playbook is a good start for anyone not already doing what I do.
AND FINALLY … THE SHOCKER OF ALL TIME
Well, it would have been a huge shocker, if any one of my medical ‘handlers’ had a good grasp of how bones really work.
It’s based on Dr. Robert Becker’s work in the 1950s-1960s, regarding the electrical nature of bones. (See: Becker, R. and Selden, G. 1998. The Body Electric: Electromagnetism And The Foundation Of Life).
Among many other things, he discovered how low DC electrical current drives bone repair.
He also pointed out the piezoelectric nature of bone. This means bones create electric charge when under mechanical stress. Did you know your bones were naturally electric? Well, they are when stressed.
Some researchers have taken that notion to a weird level. For example, the article by Tandon et al. (2018 – see references below) strives to explain how piezoelectric materials can be developed for tissue repair.
As stated in the article abstract:
The process of bone repair and regeneration requires multiple physiological cues including biochemical, electrical and mechanical – that act together to ensure functional recovery. Myriad materials have been explored as bioactive scaffolds to deliver these cues locally to the damage site, amongst these piezoelectric materials have demonstrated significant potential for tissue engineering and regeneration, especially for bone repair. Piezoelectric materials have been widely explored for power generation and harvesting, structural health monitoring, and use in biomedical devices. They have the ability to deform with physiological movements and consequently deliver electrical stimulation to cells or damaged tissue without the need of an external power source. Bone itself is piezoelectric and the charges/potentials it generates in response to mechanical activity are capable of enhancing bone growth. Piezoelectric materials are capable of stimulating the physiological electrical microenvironment, and can play a vital role to stimulate regeneration and repair.
I’m calling it weird because bones are already their own piezoelectric material! We’ve known this for nearly 70 years!
Developing piezoelectric materials to stimulate bone repair is unnecessary. The right mechanical stress is what we need. Wolff’s Law predicts it. That’s what weight-bearing exercise does for our bones (i.e., when we’re not leptin-resistant). Merely jumping up and down might even do it.
In other words, we don’t need no stinkin’ synthetic piezoelectric materials for stimulating bone repair, since that’s exactly what bones already are!
That’s It for Now
Okay, now I’m out of gas on this topic. I had fun digging into it. I hope you enjoyed learning about it as much as I did.
Comments or Questions?
I’d love to hear from you. This and every other post here provides a comment section at the end of the post, exactly for that purpose.
So, by all means, leave me your thoughts.
I promise I’ll respond in real English, not ‘science’ (at least not TOO much!).
I would be especially grateful if you point out any flaws in my logic, factual errors, or ordinary typos. (I’ll give you a little ‘huzzah’ in my heart.)
Then I’ll get back to you as soon as I can.
References
Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O, Peter JU. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD012432. doi: 10.1002/14651858.CD012432.pub3. PMID: 35866376; PMCID: PMC9309005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9309005/
Chirchir H, Kivell TL, Ruff CB, Hublin JJ, Carlson KJ, Zipfel B, Richmond BG. Recent origin of low trabecular bone density in modern humans. Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):366-71. doi: 10.1073/pnas.1411696112. Epub 2014 Dec 22. PMID: 25535354; PMCID: PMC4299206. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4299206/
Friedman AW. Important determinants of bone strength: beyond bone mineral density. J Clin Rheumatol. 2006 Apr;12(2):70-7. doi: 10.1097/01.rhu.0000208612.33819.8c. PMID: 16601540. https://pubmed.ncbi.nlm.nih.gov/16601540/
Humadi A, Alhadithi RH, Alkudiari SI. Validity of the DEXA diagnosis of involutional osteoporosis in patients with femoral neck fractures. Indian J Orthop. 2010 Jan;44(1):73-8. doi: 10.4103/0019-5413.58609. PMID: 20165680; PMCID: PMC2822423. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822423/
Lewiecki EM, Lane NE. Common mistakes in the clinical use of bone mineral density testing. Nat Clin Pract Rheumatol. 2008 Dec;4(12):667-74. doi: 10.1038/ncprheum0928. Epub 2008 Oct 21. PMID: 18936788; PMCID: PMC3891842. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3891842/
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468-89. doi: 10.1007/s001980050093. PMID: 9850356. https://pubmed.ncbi.nlm.nih.gov/9850356/
Ott SM. Bone strength: more than just bone density. Kidney Int. 2016 Jan;89(1):16-9. doi: 10.1016/j.kint.2015.11.004. PMID: 26759040. https://www.kidney-international.org/article/S0085-2538(15)00013-7/fulltext
Ryan TM, Shaw CN. Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):372-7. doi: 10.1073/pnas.1418646112. Epub 2014 Dec 22. PMID: 25535352; PMCID: PMC4299204. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4299204/
Seo SH, Lee J, Park IH. Efficacy of Dual Energy X-ray Absorptiometry for Evaluation of Biomechanical Properties: Bone Mineral Density and Actual Bone Strength. J Bone Metab. 2014 Aug;21(3):205-12. doi: 10.11005/jbm.2014.21.3.205. Epub 2014 Aug 31. PMID: 25247158; PMCID: PMC4170083. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170083/
Shafiee G, Sharifi F, Heshmat R, Ostovar A, Ebrahimpur M, Sheidaei A, Nabipour I, Larijani B. The reference value of trabecular bone score (TBS) in the Iranian population. J Diabetes Metab Disord. 2020 May 11;19(1):493-498. doi: 10.1007/s40200-020-00537-w. PMID: 32550201; PMCID: PMC7270440. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7270440/
Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018 Jun;73:1-20. doi: 10.1016/j.actbio.2018.04.026. Epub 2018 Apr 16. PMID: 29673838. https://www.sciencedirect.com/science/article/abs/pii/S1742706118302290?via%3Dihub
Vescovi P, Nammour S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A critical review. Minerva Stomatol. 2010 Apr;59(4):181-203, 204-13. English, Italian. PMID: 20360666. https://pubmed.ncbi.nlm.nih.gov/20360666/
All the best in natural health,

Statements on this page have not been evaluated by the Food and Drug Administration. Information here is not is not intended to diagnose, treat, cure, or prevent any disease.
I may receive a commission for purchases made through those links.
This doesn’t change the cost to you.
Leave a Reply